T-cell receptor (TCR) gene therapy is one of the gene-engineered T-cell therapies utilizing the potential anti-cancer reactivity of T-lymphocytes. Generally, T-lymphocytes circulate in blood and protect a body from foreign substances such as bacteria, viruses etc. On the other hand, proteins called cancer antigen are presented on the surface of cancer cells, which makes T-lymphocytes capable of recognizing and attacking these cancer cells. This therapy is one of cancer immunotherapies and is recently garnering attention as a new cancer therapy.
In this therapy, TCR genes which specifically recognize cancer antigens are transduced into T-lymphocytes isolated from a patient, and the gene-transduced cells are cultured in a large scale, and subsequently infused back into the patient. By using different TCR genes, TCR gene therapy can provide treatment for various cancers. In the manufacturing process, it is important to transduce the genes with high efficiency into T-lymphocytes, and RetroNectin® is used for this purpose. RetroNectin® is a recombinant human fibronectin fragment that promotes colocalization of virus vector with target cells to dramatically enhance transduction efficiency.
Note : As for RetroNectin® , please refer to the following link below.
https://www.takarabio.com/learning-centers/gene-function/viral-transduction/retronectin-learning-center/technology-overview
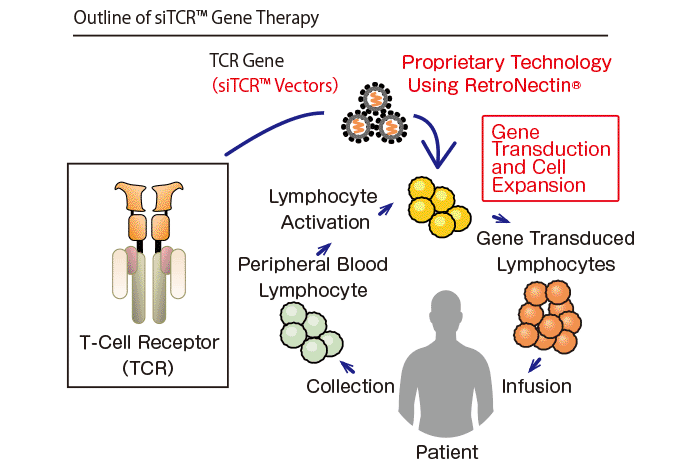
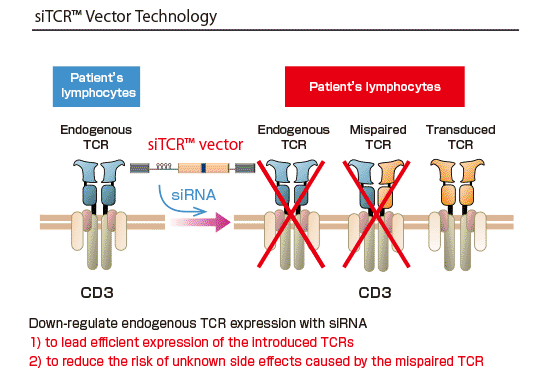
Takara Bio has been developing siTCR™ gene therapy using the siTCR™ vector technology. The siTCR™ vector technology minimizes the involvement of endogenous TCRs and allows for obtaining more T-lymphocytes that express the exogenous TCR. This is considered to reduce the risk of side effects and improve efficacy.
We have been developing NY-ESO-1 siTCR™ gene therapy for synovial sarcoma in Japan and preparing for the submission for manufacturing and marketing approval.
We are also driving forward with the development of siTCR™ gene therapies as a joint project with Princess Margaret Cancer Centre in Canada.
Cancer antigen specific TCR gene transduced T-lymphocytes exert cytotoxicity on cancer cells by the following mode of action and mediate anti-tumor effect such as tumor regression.
- Tumor antigen specific TCR is expressed on the cell surface of TCR gene transduced T-lymphocytes.
- The expressed TCR molecules recognize tumor antigens (complex of an HLA molecule and a presented antigen peptide) of target tumor cells. Subsequently, signal transduction is activated within gene-transduced T-lymphocytes, and cytotoxic activity against cancer cells is exerted by release of cytotoxic molecules.

- Kawai A, Ishihara M, Nakamura T, et al. Safety and Efficacy of NY-ESO-1 Antigen-specific T-cell Receptor Gene-Transduced T Lymphocytes in Patients with Synovial Sarcoma: A Phase I/II Clinical Trial. Clin Cancer Res. 2023;29(24):5069-5078.
- Ishihara M, Kitano S, Kageyama S, et al. NY-ESO-1-specific redirected T cells with endogenous TCR knockdown mediate tumor response and cytokine release syndrome. J Immunother Cancer 2022 Jun; 10(6): e003811.
- Tawara I, Kageyama S, Miyahara Y, et al. Safety and persistence of WT1-specific T-cell receptor gene-transduced lymphocytes in patients with AML and MDS. Blood blood-2017-06-791202, 2017.
- Kageyama S, Ikeda H, Miyahara Y, et al. Adoptive Transfer of MAGE-A4 T-cell Receptor Gene-Transduced Lymphocytes in Patients with Recurrent Esophageal Cancer. Clin Cancer Res. 21(10):2268-2277, 2015.
- Okamoto S, Amaishi Y, Goto Y, et al. A Promising Vector for TCR Gene Therapy: Differential Effect of siRNA, 2A Peptide, and Disulfide Bond on the Introduced TCR Expression. Mol Ther Nucleic Acids. 1:e63, 2012.
- Okamoto S, Mineno J, Ikeda H, et al. Improved expression and reactivity of transduced tumor-specific TCRs in human lymphocytes by specific silencing of endogenous TCR. Cancer Res. 69(23):9003-9011, 2009.
|
Conference |
Title |
|
2023 ASCO (Annual Meeting - American Society of Clinical Oncology) Annual Meeting June 2 - 6, 2023 Chicago, U.S.A. |
Results from phase I/II study of NY-ESO-1-specific TCR gene-transduced T cell therapy (TBI-1301, mipetresgene autoleucel) in patients with advanced synovial sarcoma. |
|
The 26th Annual Meeting of Japanese Association of Cancer Immunology July 20 - 22, 2022 Shimane, Japan |
T-cell characteristics associated with cytokine release syndrome (CRS) after NY-ESO-1·TCR-T cell transfer |
|
2022 ASCO (Annual Meeting - American Society of Clinical Oncology) June 3 - 7, 2022 Chicago, U.S.A. |
The addition of fludarabine to cyclophosphamide for lymphodepleting chemotherapy enhances the persistence of infused NY-ESO-1 TCR anticancer therapy TBI-1301. |
|
ESMO (European Society for Medical Oncology) 2019 Congress September 27 - October 1, 2019 Barcelona, Spain |
A novel affinity-enhanced NY-ESO-1 targeting TCR-redirected T cell transfer exhibited early-onset cytokine release syndrome and subsequent tumor responses in synovial sarcoma patients |
|
Adoptive T cell therapy with TBI-1301 results in gene-engineered T cell persistence and anti-tumor responses in patients with NY-ESO-1 expressing solid tumors |
|
|
2019 ASCO (Annual Meeting - American Society of Clinical Oncology) May 31 - June 4, 2019 Chicago, U.S.A. |
Tumor responses and early onset cytokine release syndrome in synovial sarcoma patients treated with a novel affinity-enhanced NY-ESO-1-targeting TCR-redirected T cell transfer |
|
Effect of minimal lymphodepletion prior to ACT with TBI-1301, NY-ESO-1 specific gene-engineered TCR-T cells, on clinical responses and CRS |
|
|
SITC (The Society for Immunotherapy of Cancer) 2018 November 7 -11, 2018 Washington, D.C. |
Adoptive cell transfer with NY-ESO-1 specific TCR T cells (TBI-1301) results in persistence, cytokine release syndrome and anti-tumor activity |
|
ESMO (European Society for Medical Oncology) 2018 Congress October 19 - 23, 2018 Munich, Germany |
Study of TBI-1301 (NY-ESO-1 specific TCR gene transduced autologous T lymphocytes) in patients with solid tumors |
|
The 22nd Annual Meeting of Japanese Association of Cancer Immunology August1 - 3, 2018 Okayama, Japan |
Development of cell therapy using cancer-specific T cells |
|
The 16th Annual Meeting of Japanese Society of Medical Oncology (JSMO) July19 - 21, 2018 Kobe, Japan |
First-In-Human clinical trials of TCR-engineered T cells transfer for patients with refractory solid malignant tumors |
|
The 1st Meeting of Japanese Association of Sarcoma Treatment and Research (JSTAR) |
CRS after adoptive transfer of NY-ESO-1 specific TCR-engineered T cells for patients with refractory synovial sarcoma |
|
Immunotherapy Targeting NY-ESO-1 for Sarcomas |
|
|
The 59th American Society of Hematology Annual Meeting December 9 - 12 , 2017 Atlanta, U.S.A |
Cytokine release syndrome and tumor responses in a first-in-man trial of a novel affinity-enhanced TCR-gene transduced T cell transfer targeting NY-ESO-1 antigen |
|
The 55th Annual Meeting of Japan Society of Clinical Oncology October 20 - 22, 2017 Yokohama, Japan |
Adoptive Transfer of Tumor Specific T-cell Receptor Gene-Transduced Lymphocytes - Clinical Effect and Immune Related Adverse Event (irAE)- |
|
Adoptive T cell therapy for cancer patients |
|
|
The 25th Congress of the European Society of Gene and Cell Therapy October 17 - 20, 2017 Berlin, Germany |
Cytokine release syndrome and tumor responses in a first-in-man trial using affinity-enhanced TCR-gene transduced T cells transfer in patients with NY-ESO-1(+) refractory solid tumors |
|
The 76th Annual Meeting of the Japanese Cancer Association September 28 - 30, 2017 Yokohama, Japan |
Two clinical trials of adoptive transfer of TCR-engineered T cells specific to MAGE-A4 and NY-ESO-1 |
|
Clinical response and cytokine release syndrome following adoptive transfer of NY-ESO-1 specific TCR-engineered T cells NY-ESO-1 |
|
|
The 23rd Annual Meeting of Japan Society of Gene and Cell Therapy July 20 - 22, 2017, Okayama, Japan |
Clinical trials of NY-ESO-1 and MAGE-A4 targeting TCR-gene transduced T cell transfer to patients with refractory solid tumors |
|
The 21st Annual Meeting of Japanese Association of Cancer Immunology June 28 - 30, 2017 Chiba, Japan |
Cytokine release syndrome and clinical responses after adoptive transfer of NY-ESO-1/TCR gene-transduced T cells |
|
In vitro safety evaluation, and in vivo kinetic analysis of transfused cells and serological analysis in TCR-engineered T-cell therapy targeting NY-ESO-1 |
