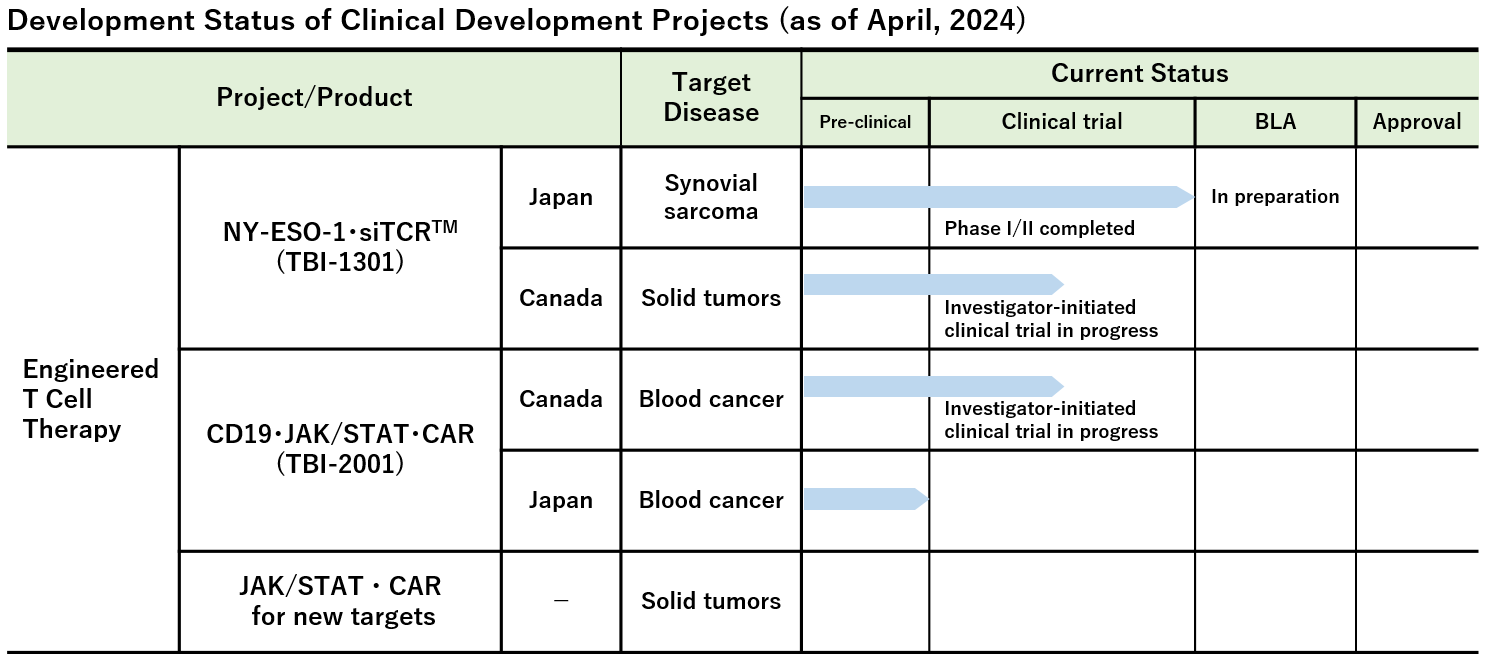
Note : We have also been advancing other investigator-initiated clinical trials and clinical research in addition to the above projects.
- Multi-center Phase I/II Study of NY-ESO-1 T Cell Receptor Gene Transferred T Lymphocytes in Patients With Synovial Sarcoma
Investigational Site: National Hospital Organization Osaka National Hospital, Mie University Hospital, Sapporo Medical University Hospital et al.
;jRCT1080223459, NCT03250325
- Multi-center, Investigator Initiated Phase 1 Study of NY-ESO-1 Specific TCR Gene Transferred T Lymphocytes With Solid Tumors
Investigational Site: Mie University Hospital, Aichi Medical University Hospital, Keio University Hospital, National Cancer Center Hospital East, National Cancer Center Hospital, and Nagoya Medical Center
;jRCT2080222844, NCT02366546
- Phase Ib Study of TBI-1301 (NY-ESO-1 Specific TCR Gene Transduced Autologous T Lymphocytes) in Patients with Solid Tumors (Investigator-initiated clinical trial)
Investigational Site: Princess Margaret Cancer Centre, Canada
;NCT02869217
- Phase I/Ib Study of TBI-2001 for Patients With Relapsed or Refractory CD19+ B-cell Lymphoma, Chronic Lymphocytic Leukemia (CLL), Small Lymphocytic Lymphoma (SLL) (Investigator-initiated clinical trial)
Investigational Site: Princess Margaret Cancer Centre, Canada
; NCT05963217
TBI-1401, Oncolytic virus C-REV
- A Phase II Study of Combination Treatment With TBI-1401(HF10), a Replication-competent HSV-1 Oncolytic Virus, and Ipilimumab in Japanese Patients With Stage IIIB, IIIC, or IV Unresectable or Metastatic Malignant Melanoma
Investigational Site: National Cancer Center Hospital et al.
;jRCT1080223536, NCT03153085
- A Phase II Study of Combination Treatment with HF10, a Replication-competent HSV-1 Oncolytic Virus, and Ipilimumab in Patients with Stage IIIB, Stage IIIC, or Stage IV Unresectable or Metastatic Melanoma
Investigational Site: Huntsman Cancer Institute, University of Utah et al.
;NCT02272855
- Phase II Neoadjuvant trial of Nivolumab in Combination with HF10 Oncolytic Viral Therapy in Resectable Stage IIIB, IIIC, IVM1a Melanoma (Neo-NivoHF10) (Investigator-initiated clinical trial)
Investigational Site: Huntsman Cancer Institute, University of Utah
;NCT03259425
- Phase I Study of Combination With TBI-1401(HF10), a Replication-competent HSV-1 Oncolytic Virus, and Chemotherapy in Japanese Patients With Stage III or IV Unresectable Pancreatic Cancer
Investigational Site: Kanagawa Cancer Center et al.
;jRCT1080223615, NCT03252808
TBI-1501, CD19・CAR
- A Multicenter Phase I/II Study for Relapsed or Refractory CD19+ B-acute Lymphoblastic Leukemia
Investigational Site: Jichi Medical University Hospital, The Institute of Medical Science, the University of Tokyo, Mie University Hospital et al.
;jRCT1080223510, NCT03155191
Please send an email to the following address
takara-clinical@takara-bio.co.jp
