Chimeric Antigen Receptor (CAR) gene therapy is one of the gene-engineered T-cell therapies, which is essentially the same as T-Cell Receptor (TCR) gene therapy. CAR is artificially constructed by combining a single chain variable fragment derived from antibodies and cytoplasmic domain(s) required for lymphocyte activation. The genetically engineered CAR-T lymphocytes recognize the antigens on the cell surface and attack the tumor cells. CAR gene therapy differs from TCR gene therapy in the structure of receptors expressed on the genetically engineered T lymphocytes. The basic steps from collecting T lymphocytes from patients, expanding them after transduction and infusing back into the patients are the same for CAR and TCR gene therapies. Takara Bio's RetroNectin® is used to obtain efficient gene transduction during the manufacture. RetroNectin® is a recombinant human fibronectin fragment that promotes colocalization of virus vector with target cells to dramatically enhance transduction efficiency.
Please refer to the following link for our RetroNectin®.
https://www.takarabio.com/learning-centers/gene-function/viral-transduction/retronectin-learning-center/technology-overview
In the CAR-T cell therapy, the structure of CAR plays an important role to determine the target cancer type and demonstrate its therapeutic efficacy. As several CAR-T cell products become available on the market, it became known that relapses due to the low persistency of CAR-T cells is a clinical challenge. Takara Bio has been developing the anti-cancer therapeutic product using a next-generation CAR technology in collaboration with Princess Margaret Cancer Centre (PM Cancer Centre) in Canada. A newly developed "JAK/STAT CAR" has a function to activate JAK/STAT signaling pathway which is one of critical pathways for proliferation and persistence of T lymphocytes (see Figure below). It has been reported that JAK/STAT CAR-T cells demonstrate enhanced antitumor activity through its increased proliferation and persistence of T lymphocytes in the preclinical study (Nat Med. 2018 24(3):352-359). This JAK/STAT CAR is currently categorized as fifth-generation CAR (Br J Cancer. 2019 120(1):26-37). Takara Bio has concluded a patent license agreement for this JAK/STAT cytokine signaling technology with University Health Network where PM Cancer Centre belongs.
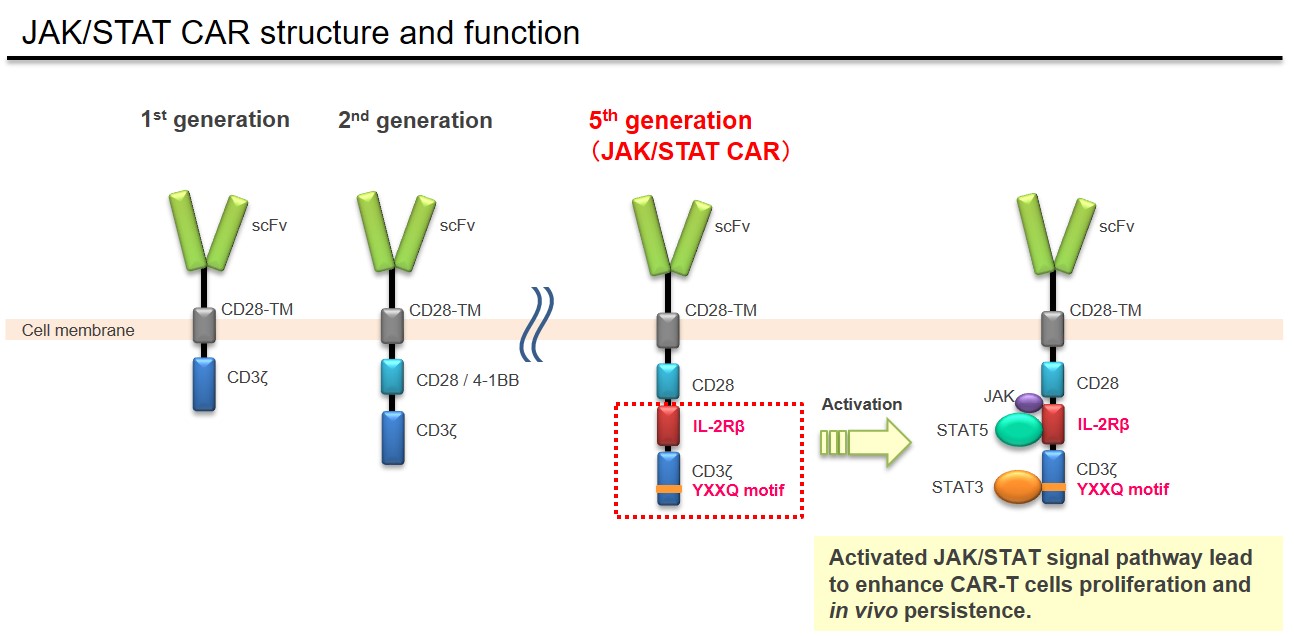
Takara Bio provides Contract Development and Manufacturing Organization (CDMO) services for gene therapy products using JAK/STAT technology and siTCR™ technology. Please refer to the following link for our CDMO service for gene therapy products.
https://ir.takara-bio.co.jp/en/news_all/news_Release/news_220908Eejg76z98b.html
Redirected CAR T-lymphocytes to the surface antigen of malignant cells exert cytotoxicity by the following steps:
- Tumor antigen specific CAR is expressed on the cell surface by transducing CAR gene into T-lymphocytes.
- The expressed CAR molecules recognize antigens on the target malignant cells. Subsequently, signal transduction is activated within gene-transduced T-lymphocytes, and antitumor activity to malignant cells is exerted by release of cytotoxic molecules.

The investigator-initiated clinical trial of CD19 JAK/STAT CAR therapy targeting CD19 positive blood cancer (Development code: TBI-2001) is ongoing at PM Cancer Centre in Canada. We are also proceeding to initiate a new clinical trial using next-generation CAR-T products which recognize the antigen expressed on solid tumor.
|
Project / Development Code |
Recognize antigen |
Signal transduction domain |
Target cancer |
Status |
|---|
|
CD19·JAK/STAT·CAR/TBI-2001 |
CD19 |
CD28-ΔIL2RB-CD3ζ (YXXQ) |
Blood cancer |
Clinical |
|
*JAK/STAT·CAR |
*Undisclosed target |
CD28-ΔIL2RB-CD3ζ (YXXQ) |
Solid tumor |
Pre-clinical |
CD19·JAK/STAT·CAR/TBI-2001
- Kagoya Y, Tanaka S, Guo T et al. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat Med. 2018 March 24(3): 352-359
|
Conference |
Title |
|
The 30th Annual Meeting of Japan Society of Gene and Cell Therapy July 16 - 18, 2024 Yokohama, Japan |
Development of anti-HER2-JAK/STAT-CAR-T therapy using short period operation for T-cell production (Spo-T) method |
|
Development of CAR-T cells that co-express Dominant negative TGFβR II and chimeric cytokine receptors |
|
|
2024 ASGCT (American Society of Gene and Cell Therapy) Annual Meeting May 7 - 11, 2024 Baltimore, U.S.A |
Enhancing the Efficacy of Anti-HER2-JAK/STAT-CAR-T Cells through Shortening the Cell Production Period |
|
Summit for Cancer Immunotherapy 2023 October 1 - 4, 2023 Canada |
Comparability and Compatibility Studies of TBI-2001: a Novel CAR T Cell Product with a JAK/STAT Signalling Domain. |
|
The 28th Annual Meeting of Japan Society of Gene and Cell Therapy July 14 - 16, 2022 Fukuoka, Japan |
Comparability assessment after transfer of the manufacturing process for a next generation CAR-T (CD19-JAK/STAT CAR-T, TBI-2001) |
