Takara Bio Inc. (“Takara Bio”) had been aiming to apply for marketing approval of its NY-ESO-1・siTCRTM gene therapy (TBI-1301; mipetresgene autoleucel, hereinafter referred to as “the product”) under the Conditional and Time-limited Approval System. Now, following discussions with the Pharmaceuticals and Medical Devices Agency (PMDA), Takara Bio has decided to revise its regulatory and development strategy, and to pursue full approval by conducting a new confirmatory clinical trial.
The product has been designated by the Ministry of Health, Labour and Welfare as an Orphan Regenerative Medical Product and is also eligible for the Sakigake Designation System (a fast-track review program for innovative therapies). Accordingly, upon completion of the confirmatory trial, Takara Bio plans to utilize the priority review process through the Sakigake Comprehensive Evaluation Consultation and submit an application for full marketing approval.
While conducting a new confirmatory trial may delay the product’s market launch compared to obtaining conditional and time-limited approval, it should expedite the process toward full approval.
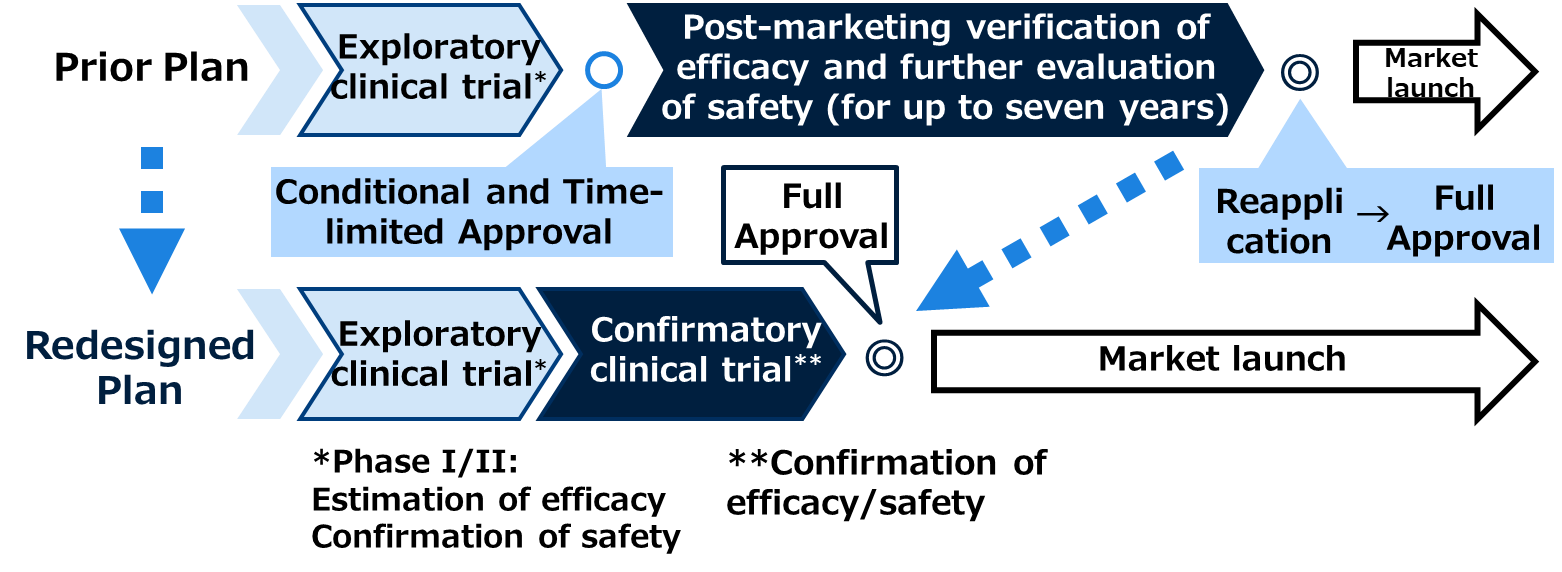
The Conditional and Time-limited Approval System for regenerative medical products allows for early marketing approval of products whose efficacy is presumed based on exploratory clinical trial results and whose safety has been confirmed. Under this system, approval is granted with specific conditions and a time limit. After market launch, further evaluation of efficacy and safety is required, and a reapplication for full approval must be submitted within the designated period. The post-marketing evaluation period may last up to seven years. However, it is generally difficult to manage post-marketing evaluations with the same rigor as clinical trials, which can make verifying efficacy challenging.
In light of these considerations, the Ministry of Health, Labour and Welfare issued the “Guidance on Conditional and Time-limited Approval for Regenerative Medical Products and the Formulation of Post-Marketing Efficacy Evaluation Plans” (Pharmaceutical Evaluation Division Notification No. 3, dated March 29, 2024), outlining key points to consider in the development of regenerative medical products under this system.
Taking these factors into account, Takara Bio has made it a priority to obtain full approval, which will enable the company to deliver the product sustainably over the long term. Takara Bio considers this is essential for providing a new treatment option for synovial sarcoma, a rare and difficult-to-treat cancer with limited therapeutic alternatives.
Takara Bio remains committed to advancing innovative gene and cell processing technologies and contributing to public health by broadly providing the technologies. Please note that the impact of this redesign is expected to be minimal on the company’s financial results for the fiscal year ending March 31, 2026.
