Takara Bio Inc. (the "Company") will begin constructing a new facility, the Center for Cell and Gene Processing III (hereinafter referred to as the "Facility"), in the head office area (Kusatsu, Shiga), to produce mRNA vaccine ingredients and enzymes for mRNA production.
This facility will be constructed with a subsidy from the Ministry of Economy, Trade and Industry's "Developing biopharmaceutical manufacturing sites to strengthen vaccine production" and our own fund. As a dual-use facility, in the event of an outbreak of an infectious disease (emergency), the Company manufactures ingredients for virus vector vaccines and mRNA vaccine, and enzymes for mRNA production in accordance with the instructions of the government. In addition, in normal times, in addition to our CDMO business, which manufactures various viral vectors for gene therapy and nucleic acid medicine, we also plan to manufacture ancillary materials, which assists manufacturing of gene therapy products, and conduct our own biologics development business.
The products manufactured in this facility for emergencies have a high degree of commonality in equipments and technologies with the products handled by our own businesses during normal times. This enables us to shift operations smoothly from normal times to emergencies.
We have focused on CDMO business for regenerative medicine/gene and cell therapy and biologics development businesses as early as possible. To date, we have commenced operations at the first Building (in 2014) and the second Building (in 2020) of the Center for Cell and Gene Processing. We have been engaged in technological development in this field, and have advanced the development of facilities that can handle a one-stop solution from the initial clinical development of diverse modalities to the production of late-stage clinical/commercial products and its quality control.
As a leading CDMO company of regenerative medicine/gene and cell therapy, we will continue to support the expanding development of pharmaceutical companies and other biotech companies and promote our biologics development business that creates new modalities. Through these businesses, we will promote the stable supply of vaccines and enzymes for mRNA production during pandemics and contribute to people's health.
|
Name/Location |
Center for Cell and Gene Processing III |
|---|
|
Products |
Emergency (during a pandemic): Ingrefdients for mRNA vaccine and virus vector vaccine, and enzymes for mRNA vaccine production
Gene therapy vectors, nucleic acid medicine, RetroNectin®, mRNA manufacturing enzymes and other ancillary materials for gene therapy, and the utilization in biologics development business |
|---|
|
Construction |
(1) Structure: 7-story seismic isolation structure, Building area: 2,650 m~{2}, Floor space: 16,400 m~{2} |
|---|
|
Construction period |
Construction started in 2024 to completed in 2027 |
|---|
|
Construction company |
JGC Corporation (Yokohama, Kanagawa) |
|---|
[Conceptual drawing of the Center for Cell and Gene Processing III]

[Plane view]
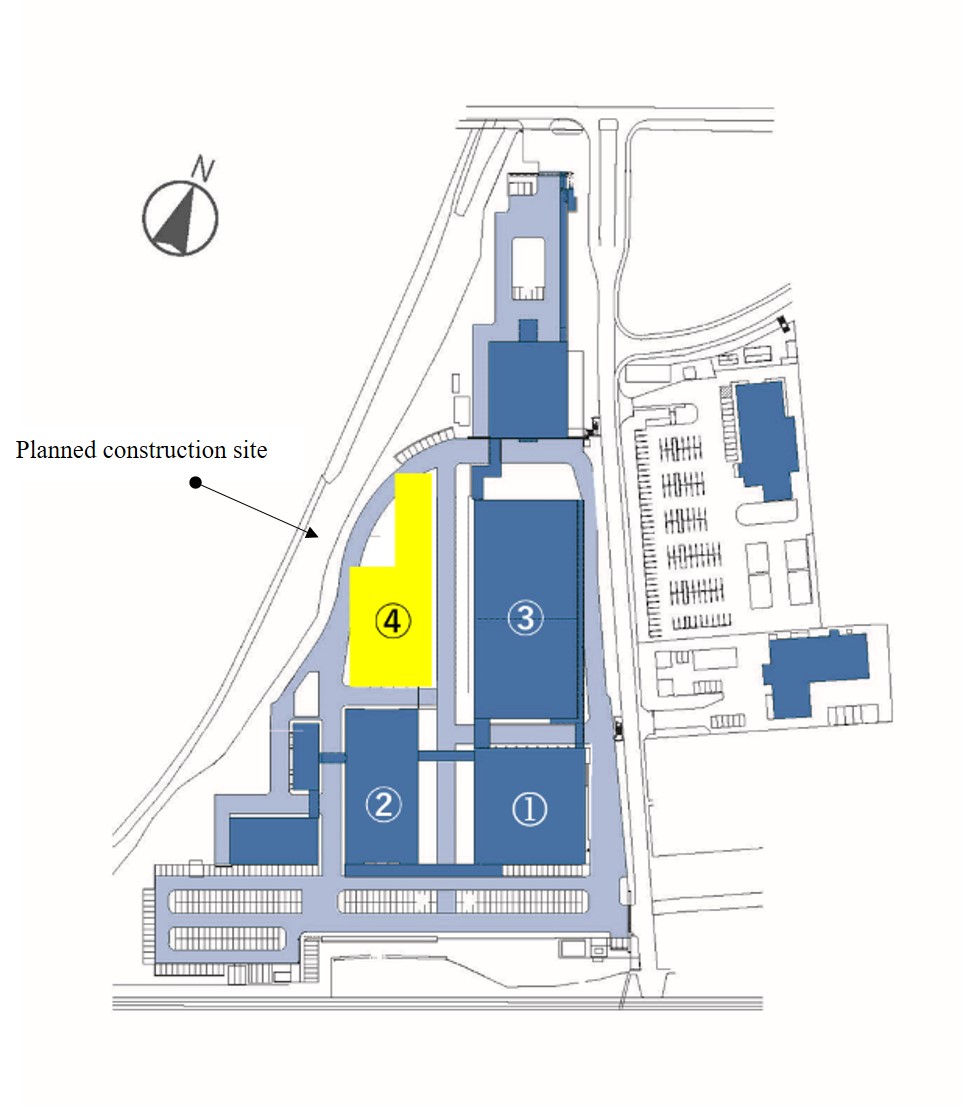
Main research and manufacturing facilities in the head office area
① Center for Cell and Gene Processing I (6,700 m~{2}) Operations started in 2014
② Gene analysis facility Operations started in 2015
③ Center for Cell and Gene Processing II (14,500 m~{2}) Operations started in 2020
④ Center for Cell and Gene Processing III (16,400 m~{2}) Scheduled to start operation in 2027
(NOTE)The area is the total floor space.
