Kusatsu / Shiga, Japan - November 9, 2021 - Takara Bio Inc. is working on the implementation of a manufacturing facility in Building 1 and Building 2 of the Center for Gene and Cell Processing (CGCP) in the head office area (Kusatsu, Shiga) that (1) flexibly accommodates a variety of modalities (Note 1), including regenerative medicine products such as gene therapy drugs and vaccines; and (2) aims to develop a domestic supply chain for important reagents, including PCR testing reagents.
In particular, when Building 2 of the CGCP went into operation in 2020, approximately one-third of the total area was designated as an expandable area that can be used to flexibly respond to various future needs. Based on the policies set forth in (1) and (2) above, we have decided the details of the complete implementation. The facilities will be put into operation upon completion, and by the spring of 2023, we plan to move up the initial plan and put them into full operation.
(1) Responding to diverse modalities
With the development of biotechnology, the usefulness of various modalities has been proven, and pharmaceutical companies and bio-ventures are promoting development with the aim of early commercialization.
The Company is preparing to commercialize NY-ESO-1・siTCR~{TM} gene therapy, which is being jointly developed with Otsuka Pharmaceutical Co., Ltd. In addition to the production of DNA vaccines for the prevention of COVID-19 carried out by AnGes, Inc. and others, and of mRNA vaccines for clinical study conducted by VLP Therapeutics Japan G.K., we are expanding our facilities to support as CDMO (Note 2) the development and manufacture of regenerative medicine products such as gene therapy drugs carried out by pharmaceutical companies and bio-ventures.
In addition to our own funds, we have received support from the Ministry of Health, Labour and Welfare's “Project for Emergency Development of Vaccine Production Systems.” The facilities are scheduled to start running in April 2022. Vaccine production will be a priority during a pandemic while they can be used flexibly during normal times for operations of the Company (dual-use). The Company will make effective use of this facility.
[Modality handled by the Company]
|
Classification |
Modality (treatment) |
|---|
|
DNA |
DNA vaccines, Gene therapy drugs, Raw materials for viral vector |
|
RNA |
mRNA vaccines, Gene therapy drugs |
|
Proteins |
Functional Protein: Gene transfer agent (RetroNectin®) |
|
Viral vector |
Gene therapy drugs, Raw materials for cell processing, vaccines |
|
Cells |
Cancer immunotherapy drugs, Regenerative medicines, Gene therapy drugs |
[Major facilities]
|
Classification |
Equipment |
|---|
|
DNA・RNA |
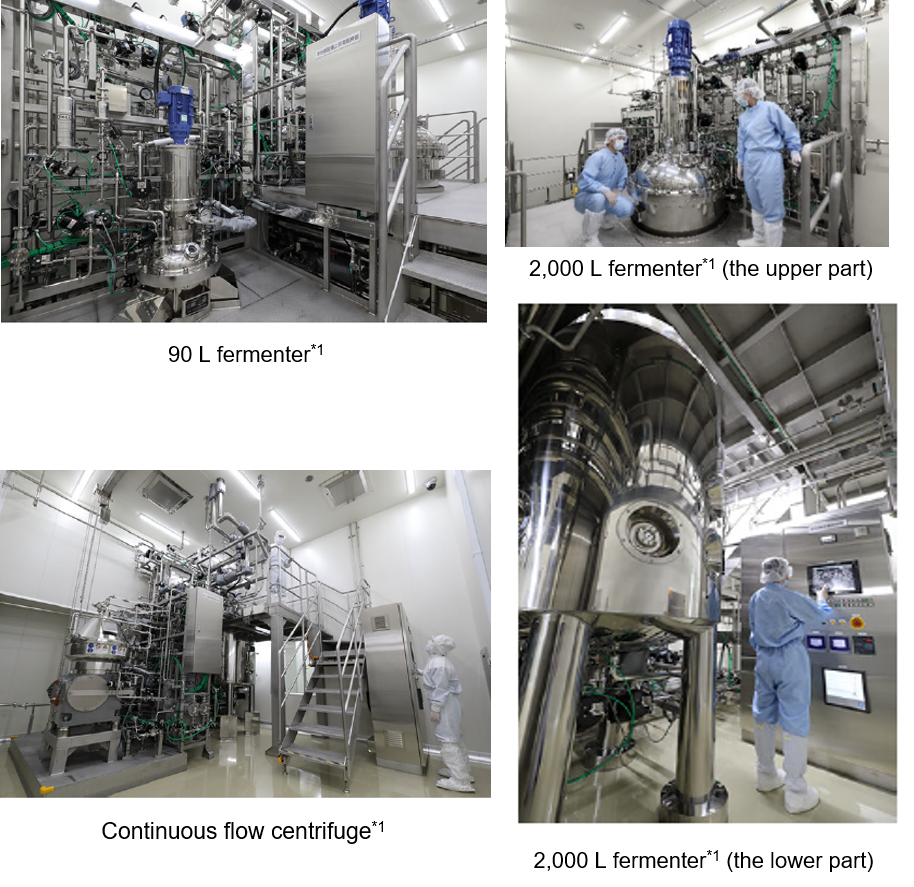
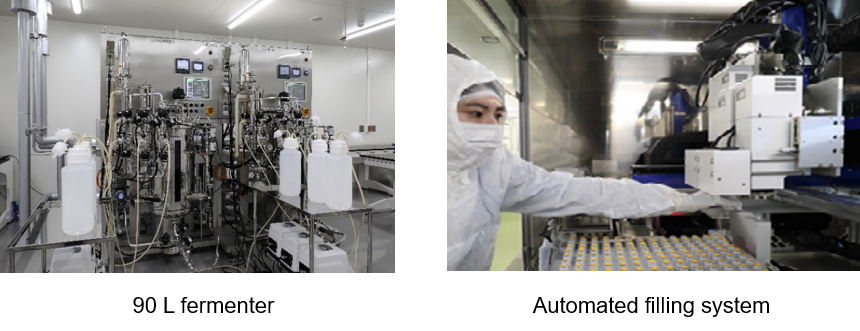
25 L rocking type shaking bio reactor (2 units), 200 L single-use bio reactor (3 units), and 90 L and 2,000 L fermenter (2 units for each)*1, continuous flow centrifuge (2 units)*1, large purification system (6 units)*1 and medium purification system (3 units) |
|
Viral vector |
Suspension culture:
|
|
Cells |
Manufacturing suite (14 suites)*3, closed-system automatic culturing system (1 unit), CO2 incubator (40 units), biosafety cabinet (31 units) |
|
Others |
Automated filling system (20,000 vials/day, 3,000 vials/day) (1 unit for each) |
*1 Covered by the support from the MHLW’s “Project for Emergency Development of Vaccine Production Systems.”
*2 Each one of 200 L and 2,000 L culture tank are scheduled to be added by 2022.
*3 Eleven suites are in operation. Additional 3 suites added during 2023. Three suites are in operation at the Center LIC branch office of the CGCP (Kawasaki, Kanagawa)
(2) Supply chain development of PCR detection reagents and other test reagents
A series of pieces of equipment, including fermenter, protein purification system, and automated packaging system, was completed in part (approximately 2,400 m~{2}) of the expanded area of Building 2 of the CGCP. It will be used for the production, storage, and shipment of COVID-19 PCR test reagents (including in vitro diagnostics) and critical reagents. Operations commenced in October 2021. Development of the facilities has been selected by the Ministry of Economy, Trade and Industry as a “Project to Promote Domestic Investment for Supply Chain Measures,” and we will utilize it.
Once development is completed, the production capacity will be a maximum of 8 million reactions per month in terms of PCR testing, enabling the Company to secure a domestic supply chain for reagents such as PCR testing during a pandemic.
[Major facilities]
[Definition of terms]
*1 Modality
It is a means of treatment. In the early stage of pharmaceuticals, low-molecular compounds were the mainstream, but thanks to advances in technology, pharmaceuticals using biological components such as genes, cells, and antibodies are now appearing.
*2 CDMO
It refers to the business of being commissioned a process from manufacturing process development to manufacturing by pharmaceutical companies.
The Company is particularly focusing on regenerative medicine products such as gene therapy drugs.
[Related news releases]
・Takara Bio Launches a New Facility for the Research and Manufacturing of Regenerative Medicine Products (January 21, 2020)
https://ir.takara-bio.co.jp/en/news_all/news_Release/news_Release201889409321505118082820200121.html
[Takara Bio (Head Office Area)]
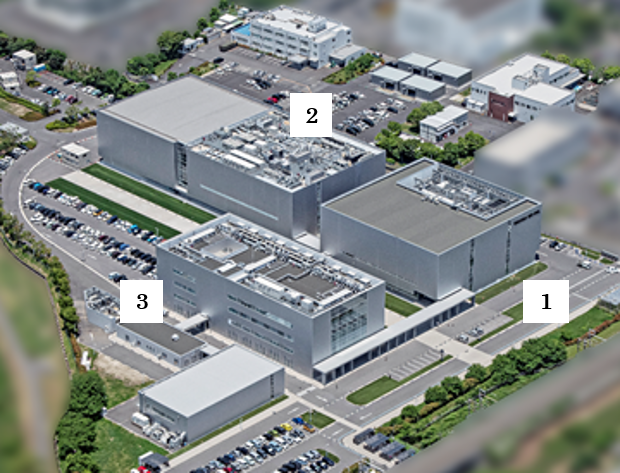
1 CGCP Building 1 (started operation in October 2014) with a floor area of 6,700 m~{2})
2 CGCP Building 2 (started operation in January 2020) with a floor area of 14,500 m~{2})
3 Main Office (started operation in August 2015)
